Weekly Versus Three Weekly Administration of Paclitaxel as Neoadjuvant Chemotherapy in HER2- negative, Stage III Breast Cancer: A comparison of treatment response
Authors: Shourov Biswas, Md. Masudur Rahman, Sarwar Alam, Mostafa Sanaul Haque, Mohammad Jahan Shams, Ekram Bin Faruque, Soma Banerjee
Institution: BSMMU
Introduction
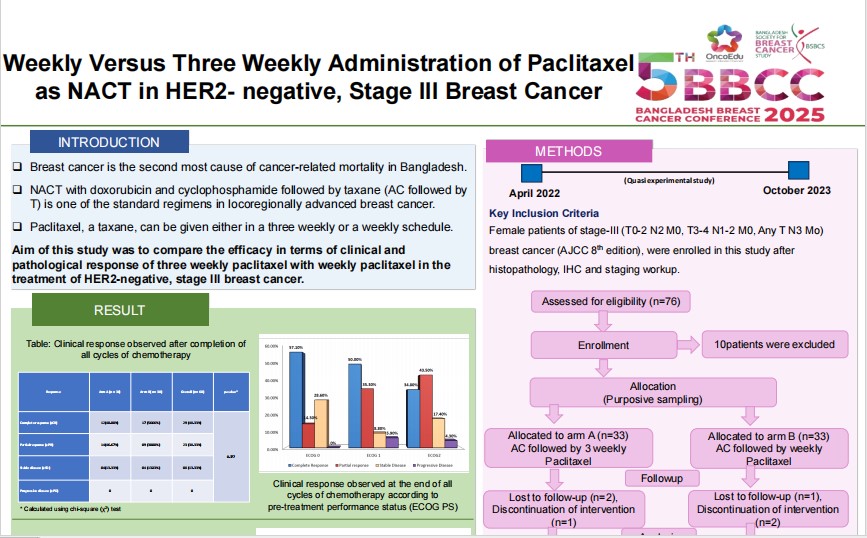
Neoadjuvant chemotherapy with doxorubicin and cyclophosphamide followed by taxane (AC followed by T) is one of the standard regimens in locoregionally advanced breast cancer. Paclitaxel, a taxane, can be given either in a three weekly or a weekly schedule. Aim of this study was to compare the efficacy in terms of clinical and pathological response of three weekly paclitaxel with weekly paclitaxel in the treatment of HER2-negative, stage III breast cancer.
Methods
A Quasi-experimental study was conducted from April 2022 to October 2023 in two centers of Dhaka, Bangladesh. Sixty-six stage-III breast cancer patients were enrolled and divided equally into two arms. Arm-A patients received four cycles of AC followed by paclitaxel (175 mg/m2) three-weekly for four cycles. Patients in arm-B received four cycles AC followed by paclitaxel (80mg/m2) weekly for 12 weeks. Patients were evaluated before, during and after the completion of the chemotherapy to assess clinical outcomes and was assessed for pathological response after surgical management.
Results
Pathological complete response (pCR) was achieved in 15 (25%) patients. Four patients (13.33%) in arm A and 11 patients (36.66%) in arm B had pCR. The difference was statistically significant (p 0.037). In both arms, patients with triple negative receptor status had increased pCR (22.22% or 2 patients out of 9 in arm A, and 85.71% or 6 patients out of 7 in arm B) in comparison to hormone receptor (ER and/or PR) positive tumors (9.52% or 2 patients out of 21 in arm A, and 21.74% or 5 patients out of 23 in arm B). But these differences were not significant.
Conclusion
After three-weekly administrations of four cycles of AC, the weekly administration of paclitaxel was found to have better pathological complete response rate in comparison to three-weekly administration of paclitaxel.