In Silico investigation on the inhibitory activity of compounds found in Manuka Honey against MCF-7 cancer cell line and design of potential lead compounds by pharmacophore modeling
Authors: Kazi Masrukh Rahman Shirsaw, Empiat Jahan Hazari Efti, Sumaiya Akter, Lutful Haque Saran*, Mst. Rozina Parul
Institution: Gono Bishwabidyalay (University), Nolam, Savar, Dhaka-1344
Introduction
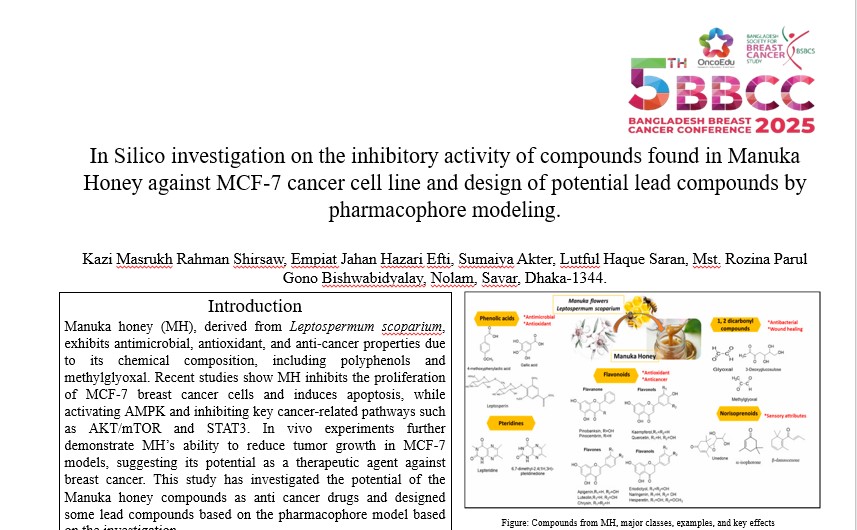
Manuka honey, derived from Leptospermum scoparium, is valued for its rich chemical composition, particularly its polyphenols, flavonoids, glyoxal, and methylglyoxal, which contribute to its antimicrobial, antioxidant, and anti-proliferative properties. In recent studies, Manuka honey (MH) demonstrates significant antitumor activity in preclinical models, particularly by inhibiting the proliferation of MCF-7 breast cancer cells in a dose-dependent manner, comparable to tamoxifen and selective toxicity sparing normal mammary epithelial cells. MH induces apoptosis in MCF-7 cells via PARP activation and modulates key molecular pathways by activating AMPK while inhibiting AKT/mTOR and STAT3 signaling, suggesting potential anti-inflammatory and tumor-suppressive mechanisms. Several recent in vivo studies further support MH's efficacy, as it significantly reduces MCF-7 tumor growth in nude mice, highlighting the need for further research into its therapeutic potential and molecular mechanisms. In this study, a thorough computational investigation has been done on the effects of Manuka honey components against the MCF-7 cells related to the pathophysiology of breast cancer, and some promising lead compounds has been designed by using pharmacophore modeling.
Methods
In silico investigation of the components of Manuka honey along with the standard drug Tamoxifen against MCF7 cells, i.e., ERα, PARP, AMPK, S6, and STAT3, includes the molecular docking of the components in PyRx, alignment of the components containing the best docking scores in PyMOL, pharmacophore modeling by the Pharmit web server, and searching of similar molecules based on pharmacophore in the ZINC database of purchasable molecule. Screening of the molecules in the ZINC database has been done by testing drug likeliness in the RPBS Mobyle web server. Pharmacokinetic and toxicological screening of the molecules have been done on the ADMET Lab 2.0 web server. Finally, the promising molecule has been docked again in PyRx against the aforementioned protein for final screening.
Results
About 28 different molecules extracted from Manuka Honey has been identified from several literatures, and their structures have been collected from Pubchem web server. Protein molecules have been collected from RCSB PDB (PDB ID for ERα: 1A52, S6:1RIS, PARP: 1UK0, p-STAT3:6NJS, and AMPK: 7MYJ). Docking results have shown that, several molecules have shown better binding affinity while dockined against RIS (-6 to -6.4), 1UK0 (-7.3 to -7.5), 6NJS (-7.5 to -8.5) and 7MYJ (-8.3 to 9 than the standard drug Temoxifen (-5.4,-7.0,-6.9 -7.2) on the other hand, compounds of MH have shown slightly lower (-7.2 to -8.5) binding affinity than Tamoxifen while docked against 1A52 (-9.2). Based on the docking scores, the top 3 molecules have been aligned in PyMOL and brought to Pharmit web server for pharmacophore modeling. Using the pharmacophore model, the ZINC database of purchasable molecules has been searched, and 44 molecules have been screened. All the 44 molecules were checked in RPBS Mobyle server for drug likeliness properties like Lipinski’s rule of 5, human, intestinal absorption, and several other properties. However, 7 molecules have passed the drug likeliness properties, and further, they were subjected to checking ADMET properties in ADMET Lab 2.0 web server, where all the molecules have been termed accepted by the server. All the 7 molecules have re-docked against those 5 protein molecules, and all molecules have shown better results in all the proteins except 1A52 than the Manuka honey molecules, and the binding affinity of top docked compounds is as follows: 1UK0 (-11.9), 6NJS (-8.7), 7MYJ (-10.4), 1RIS (-7.1), 1A52 (-8.3).
Conclusion
Manuka honey molecules have shown antitumor activity in recent in vitro and in vivo studies, supporting the in silico findings of this research. A pharmacophore model was designed, identifying seven promising lead compounds. Further in vitro and in vivo studies are needed to develop these leads into potential anti-breast cancer drugs.